The Vaccine Race
December 10, 2016
Following the Ebola virus frenzy of 2014, the Zika virus dominated much of 2016. In February of 2016, the World Health Organization (WHO) categorized Zika as a “Public Health Emergency of International Concern,” adding it to the growing list of historic epidemics.
Although the WHO has since descaled the severity of the Zika virus, it remains a growing concern among public health experts. People infected by the virus may not always be symptomatic, but Zika is responsible for a growing number of microcephaly cases, which result in crippling birth defects. Microcephaly, an extraordinarily rare condition affecting only two in 10,000 babies in the United States is characterized by the unusually small head of a baby, leading to other complications like seizures and developmental delay. For this reason, it is imperative to expedite the creation of a vaccine to inhibit the spread of Zika.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, called a meeting between a number of pharmaceutical corporations, including GlaxoSmithKline, Inovio Pharmaceuticals, NewLink, and Sanofi to discuss the potent threat of Zika. “I said, all hands on deck,” Dr. Fauci recalled. “We have a serious problem here. We’ve got to really move.”
A number of pharmaceutical companies, in the pursuit of finding such a vaccine, have prioritized developing a strain which would immunize humans from the virus. In this quest, corporations ranging from the Japan’s Takeda to France’s Sanofi are working at an incredibly fast pace. In fact, projections for the approval of public use vaccines are set for 2018.
In addition to private sector efforts, President Obama proposed a $1.9 billion fund in February of 2016 to further the development of the vaccine and facilitate testing for Zika in pregnant women. However, his proposal faced multiple roadblocks in Congress due to partisan divisions. Republicans changed the bill to include restrictions on family planning and Obamacare, which Senate Democrats rejected. A slightly adjusted bill with $1.1 billion in funding ultimately passed in late September of 2016.
From Catherine Chen’s ’18 perspective, “The Zika vaccine is being developed so quickly through the incredible collaboration between private companies as well as the government. It’s remarkable to think that by the time we graduate, a Zika vaccine might already be approved and open to the public.”
Unlike the black plague, which took place in an era with minimal access to medicine, diseases in the 21st century are becoming much easier to create quick fixes for. Anthony Lan ’20 sees the reaction to the outbreak as an inspiring matter: “I think technological advances within the field of medicine are truly making the world a better place, increasing the human life span.” The scientific world’s rapid response to the Zika virus indicates a positive trend in the effort to prevent diseases.

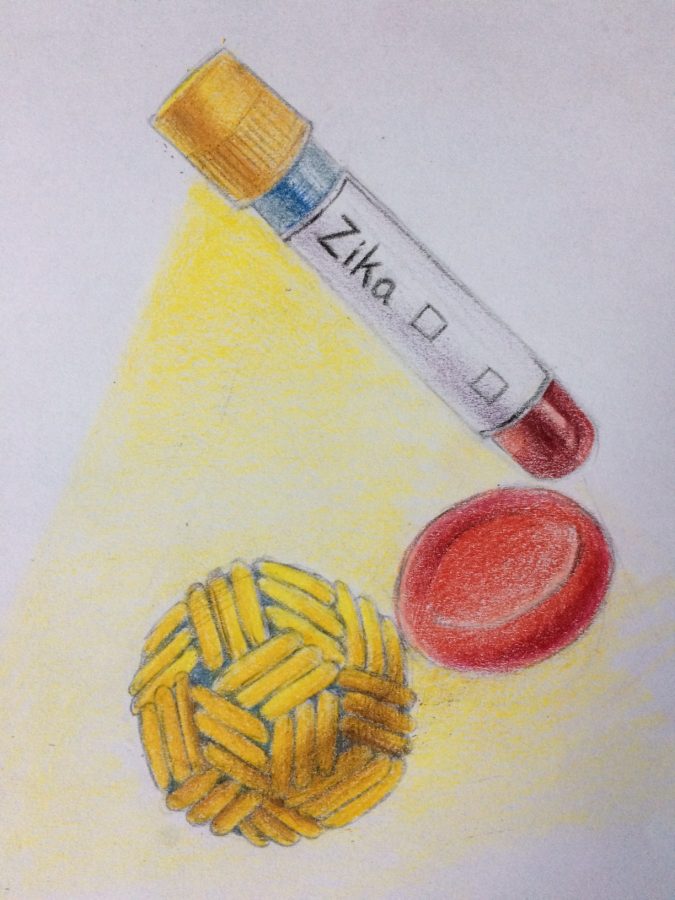

CC • Dec 20, 2016 at 2:51 pm
Although the Zika virus frenzy has relatively died down, it still remains of concern for many countries. It’s wonderful that a vaccine will be developed soon!
Camille SHen • Dec 20, 2016 at 12:42 pm
I think this was a really great article. It was very informative and I went away with a lot more knowledge about the vaccine race than I did before. 10/10 would reccomend!
Nastassja Kuznetsova • Dec 20, 2016 at 12:39 pm
Great jo on the article. Vaccines are deifnitwly crucial for herd immunity and should be made mandatory.